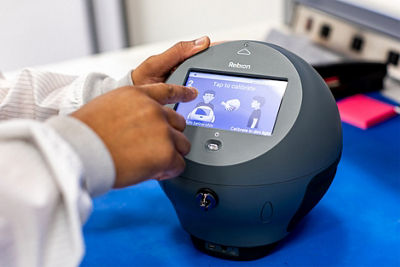
Canon Virginia, Inc. Serves as Rebion’s Manufacturer of the blinq.® Vision Scanner
NEWPORT NEWS, VA, January 28, 2021 – Canon Virginia, Inc. (CVI) and Rebion announce production for Rebion’s blinq.® vision scanning technology, the first FDA cleared device designed to detect micro-strabismus and amblyopia (lazy eye) in children ages two through eight. With its portable design, blinq.® offers an easy and efficient method for pediatricians and front-line vision screening organizations to screen children, obtain immediate results, and support efforts to eradicate a condition that impacts three to five percent of the population.
Canon Virginia first announced medical device contract manufacturing along with ISO 13485 certification in 2017, and publicly announced collaboration with Rebion in February 2020 at the PLASTEC West expo in Anaheim. “We’re extremely excited to work with CVI,” said Justin Shaka, CEO, Rebion. “Our comfort with CVI’s engineers and operators was immediate. The ease of collaboration has given our R&D and sales teams the confidence to successfully expand our market presence with high quality products.”
CVI has dedicated resources, including a state-of-the-art facility with 30,000 square feet of floor space to support Class I and Class II medical device manufacturing. CVI also has a technical staff specializing in a wide range of engineering disciplines and possessing a broad scope of skills to produce everything from single parts to complex mechanisms for medical imagery, optics and microfluidics.
“Canon Virginia is looking for collaborations with innovative, inspiring and determined medical device companies within the industry; we found this with Rebion. We believe they have worked tirelessly to address amblyopia in children and continue the development of medical products in areas that may have a lasting impact for us all,” said Toru Nishizawa, President and CEO, Canon Virginia. “I am very proud to be a part of bringing blinq.® to the market.”
CVI has expanded its collaboration with Rebion by providing video and imaging expertise and manufacturing know-how for Rebion’s Head & Intraocular Trauma Tool (HITT™), which leverages a proprietary technique known as Neural Performance Scanning, to identify brain impairments related to traumatic brain injury. The HITT device has shown promising results in preliminary studies, and will be entering its pivotal studies for market clearance soon.
“We’re bringing technologies to the market that can eliminate or reduce the consequences of brain related impairments,” said Jeff Mortensen, Executive Vice President, Rebion. “Collaborating with CVI and their expanded resources – Canon Financial Services (CFS) and Canon Information Technology Services, Inc. (CITS) – ensures our customers can secure funding for immediate acquisition of the latest market offerings and be confident with this industry-leading customer support.”
“Canon Virginia is excited to bring more than 30 years of engineering expertise and its full access to established resources to the medical industry. We are providing tailored solutions to meet Rebions’s needs,” explains Ron Kurz, Senior Director & Operations Manager of Medical Business. “Our customers inherit the precision and quality expertise of the Canon brand. Additionally, Canon Virginia brings a highly developed quality-management system that is applied to every process.”
About Canon Virginia, Inc.
Located in Newport News, Va., Canon Virginia, Inc. serves as the Canon manufacturing, engineering, recycling and technical support center for the Americas. Canon Virginia produces new products using advanced manufacturing methodologies while also serving as a factory service center providing expert customer service in the repair and refurbishment of Canon products. Canon Virginia’s manufacturing services extend to injection mold making, contract manufacturing, medical contract manufacturing and aftermarket services. For more information, call 1-866-99-CANON or visit www.cvi.canon.com.
About Rebion
Rebion manufactures innovative instruments for definitively identifying life-altering diseases that manifest from functional impairments in the brain. The company was found in 2009 by David Hunter, MD PhD, Ophthalmologist-in-Chief at Boston Children’s Hospital after more than 15 years of R&D and clinical testing at major medical institutions. Rebion was the recipient of an honorable mention award in Fast Company’s 2020 World Changing Ideas Awards. For more information, visit https://www.rebion.net/blinq.
# # #
† Based on weekly patent counts issued by United States Patent and Trademark Office.